Our Product
High Level Overview
The Cytek Northern Lights-CLC is a new clinical full spectrum flow cytometry system that maximizes the operational efficiency of clinical laboratories across the board. It brings the same revolutionary spectral technologies to the clinical environment. Full spectrum flow cytometry is especially suited for clinical applications since it allows more information to be gleaned from a single tube, saving time, resources, and precious patient samples.
SpectroFlo® software offers an assortment of efficiency-enhancing tools, including portable templates, reusable reference controls and much more.Remarkable Sensitivity and Data Quality
Clinical Insights with Less Sample
Ease of Use
Service Plan Comparison
Service Contract Types
Gold
Includes
- Unlimited Coverage Of Parts, Labor & Travel
- 1 Preventative Maintenance Visit/Year
- Priority Response Time
- Unlimited Phone & Email Support
Silver
Includes
- Limited Coverage Of Parts, Labor & Travel
- 1 Preventative Maintenance Visit/Year
- 1 Fully Covered Repair Visit
- Unlimited Phone & Email Support
- 20% Parts Discount
Bronze
Includes
- 1 Preventative Maintenance Visit/Year
- Unlimited Phone & Email Support
- 10% Parts Discount
- All Other Services Billed At Prevailing Rate
Performance Data
Deep Immunoprofiling
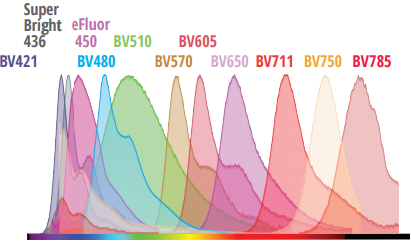
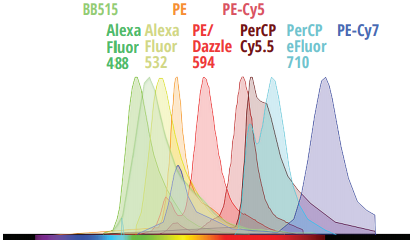
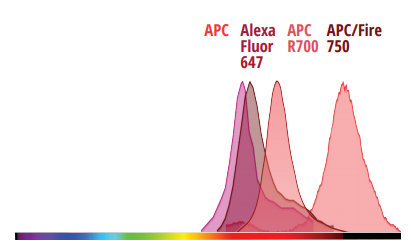
Evaluating Minimal Residual Disease (MRD)
By enabling deeper biological insights from each sample, the Northern Lights-CLC platform improves efficiencies across the entire sample-to-answer workflow for immunophenotyping, hematology, and more.
To demonstrate these efficiencies, check out this very well-established 3-tube acute myeloid leukemia (AML) MRD assay1 created for a conventional 3-laser (405, 488, and 638 nm) flow cytometer. To complete the assay, 3 separate tubes must be prepared and acquired. With Cytek’s Northern Lights-CLC, the same information can be obtained from a single tube.1. Wood, B. L. (2020). Acute Myeloid Leukemia Minimal Residual Disease Detection: The Difference from Normal Approach. Current Protocols in Cytometry, 93(1). doi:10.1002/cpcy.73
Submit this form to download the white paper
Complete the fields below to download the white paper Creating Efficiency in the Lab with Full Spectrum Cytometry. Check the consent boxes at the end of the form to receive additional communication from the Cytek Biosciences team.
![Cytek® Northern Lights-CLC [Flow Cytometry System]](https://cytek-web.s3.amazonaws.com/cytekbio.com/instruments/instr.nl-clc.evaluating-MRD-graphic.png)





