Why is Multi-Site Setup Standardization Important?
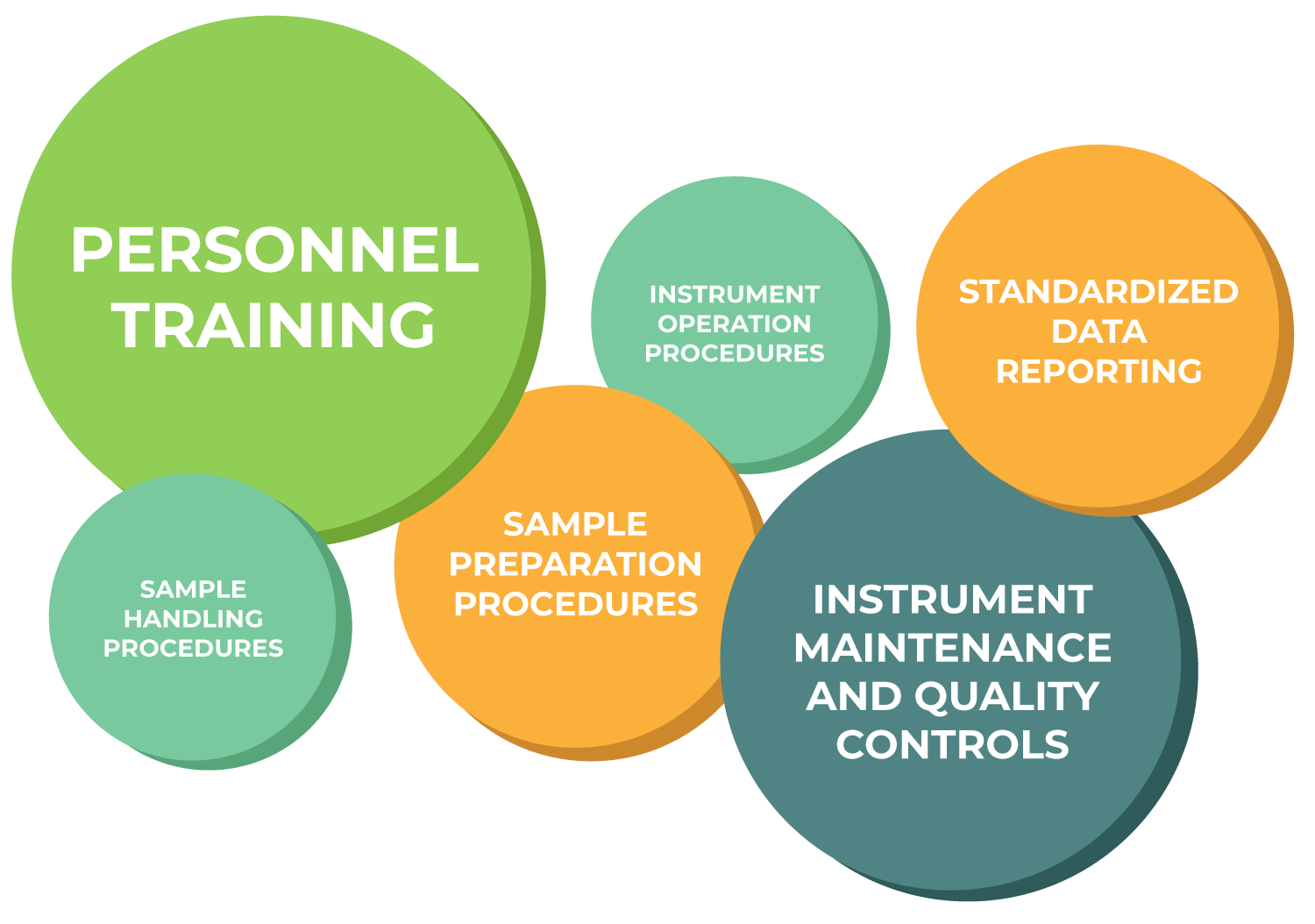
Whether running a clinical trial to evaluate efficacy for a new drug or screening patients for disease, clinical research organizations (CROs) require controlled methods and procedures to minimize variation across sites and obtain meaningful and reliable results.
Cytek provides instruments that can be standardized to reduce instrument to instrument variability, enabling reproducibility of data across any site. In addition to the instrument itself, Cytek-developed software, accessories, and site services can further help CROs achieve standardization and harmonization across different sites around the world.
Q2 Solutions Announces Deployment of Next Generation Flow Cytometry Services
Standardizing an Assay Across Labs
After installing the same configuration of the Cytek Aurora at each lab, assay creation and setup standardization can begin. The assay must first be designed, optimized, and validated on a single instrument with Cytek’s pre-optimized instrument settings (CytekAssaySetting) to verify performance. After the assay is generated, an experiment template complete with an analysis worksheet is saved and shared with the other labs. The template is imported onto the other Cytek Auroras, and the assay is ready for use across sites.

